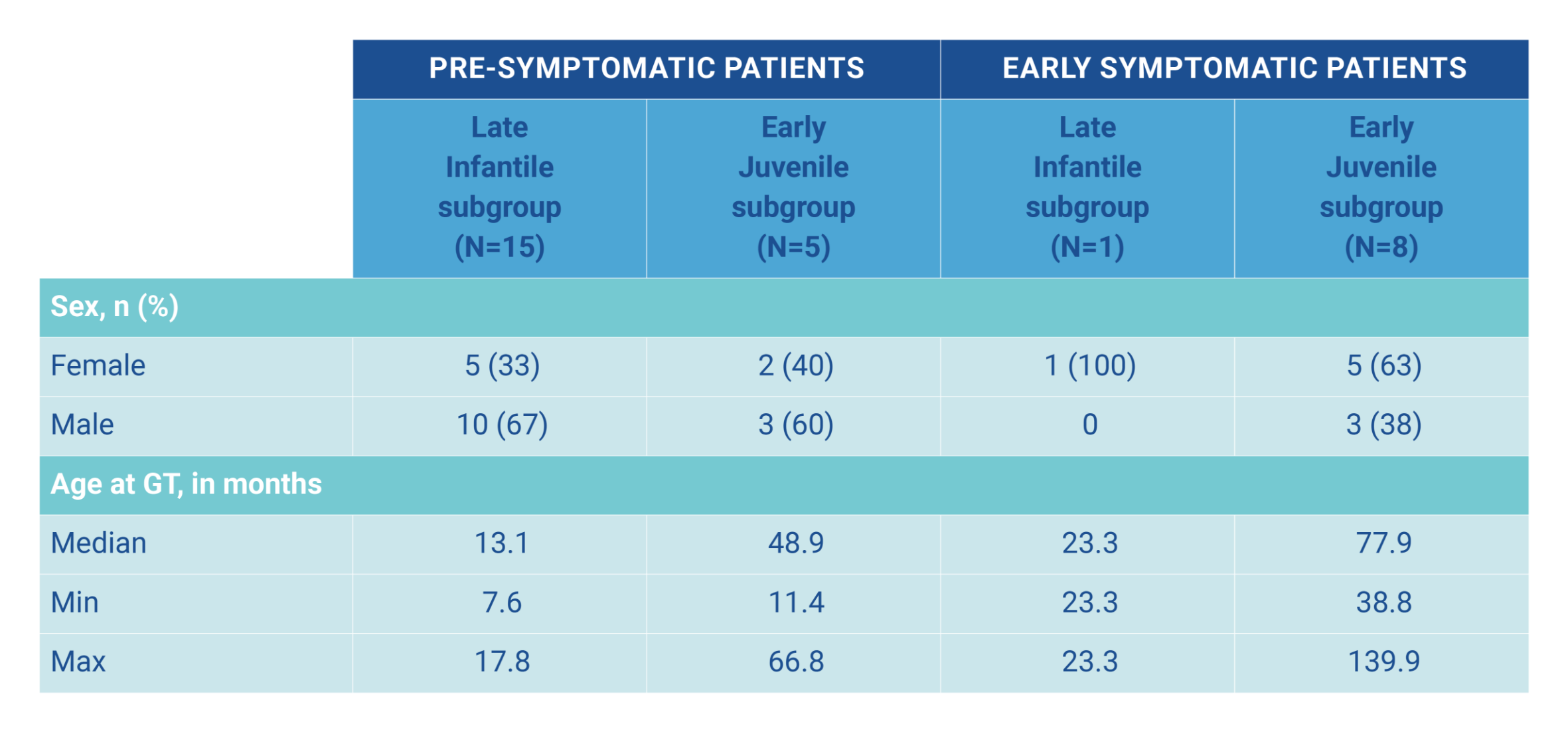
In an integrated data set, 29 children with MLD (16 late infantile, 13 early juvenile) aged 7.6‒139.9 months were treated with Libmeldy as part of the safety analysis and 25 children with MLD were included in the efficacy analysis. They were compared with 31 untreated children with MLD (19 late infantile, 12 early juvenile). The median duration of follow-up was 3.16 years (range 0.64-7.51 years). The aim of the study was to determine the efficacy and safety of Libmeldy hematopoietic stem cell (HSC) gene therapy. Of the 29 patents included (who were given the fresh formulation (OTL0200-f), 20 were from Study 201222 (Registrational study), 1 was from CUP 207394 (Compassionate Use Programme), 3 were from HE 205029 (Hospital exemption) and 5 were from SUP 206258 (Compassionate Use Programme).1

Libmeldy Summary of Product Characteristics.2

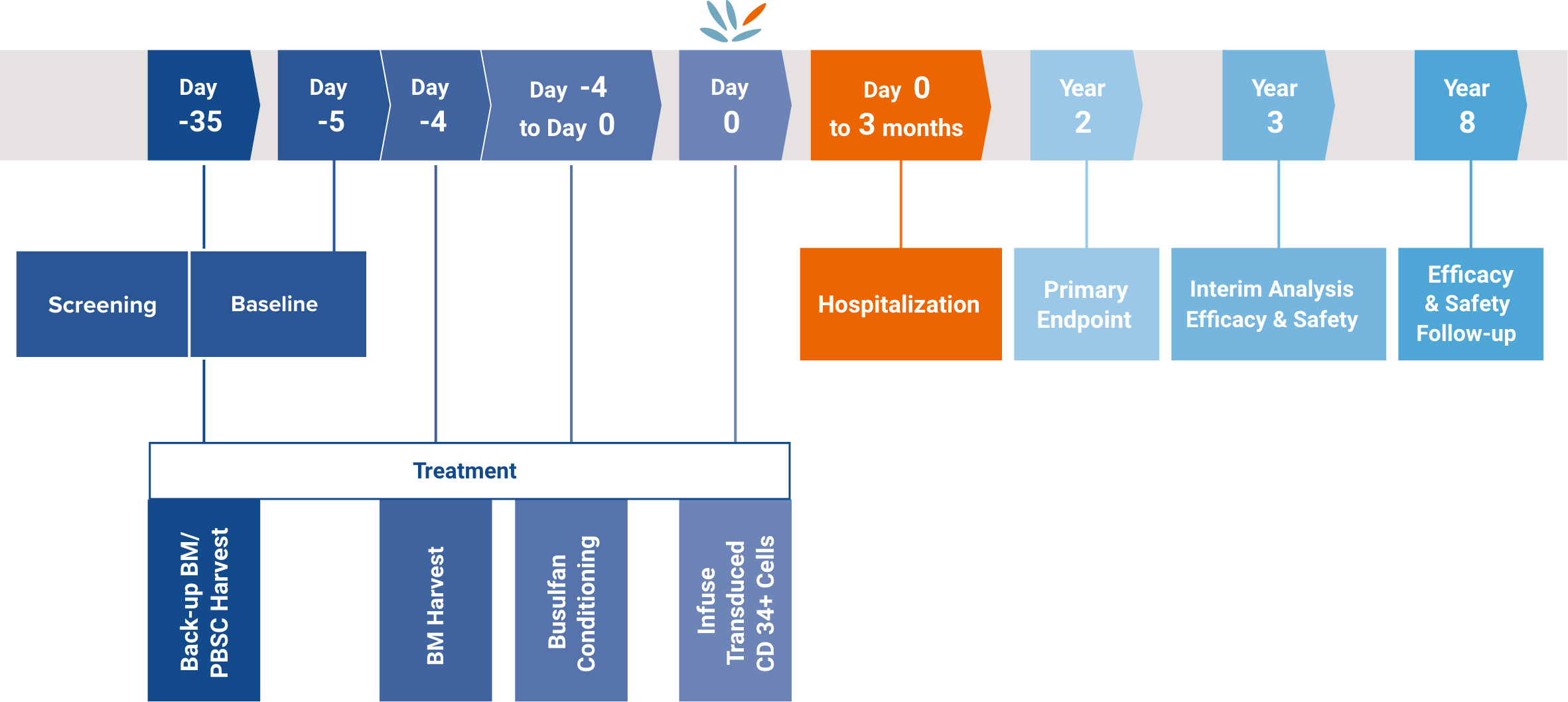
The co-primary endpoints were, at 2 years post-treatment:3,4
At year 3, an interim safety and efficacy analysis was planned and, at year 8, an efficacy and safety follow-up was planned.

Abbreviations
GMFM, gross motor function measurement; PBMC, peripheral blood mononuclear cells
References